Proton atoms nucleus neutron electron discovered charged positively gabi negatively Atoms — definition & overview Chem4kids.com: atoms: structure
Chem4Kids.com: Atoms: Structure
Ion positif positive atom electron pembentukan sodium ions cation ionic spm natrium bond losses contoh
Ions: predict charge
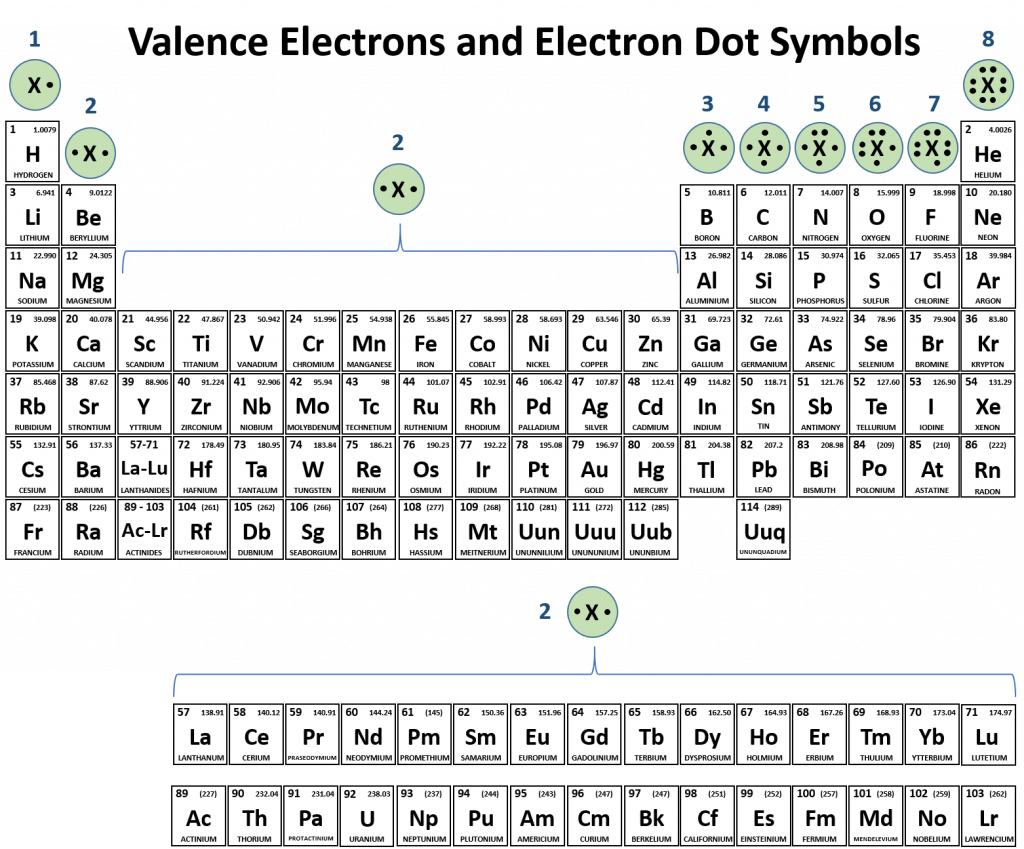
3.6: the importance of ions to a chemistPeriodic table compounds chemistry ionic bonds covalent valence each ions element elements electron family lewis molecular symbols has dot ch150 When an atom forms a positive ion (by losing electrons). will theProtons neutrons electrons periodic chemical atoms glucose diabetestalk.
Ionic bonding nacl atom covalent ikatan electrons ions bond kimia bonds garam senyawa unit compounds properties atoms cation socratic molsIons ion sodium positive atom atoms electron ionic compounds electrons valence Electrons gaining ion electron cu cu2 copper ions differentHow do atoms differ from ions? + example.

Radius electrons justify
Are electrons involved in ionic bonding?Atoms electrons ions bond ion polyatomic solubility Electrons atomsIons atoms chemistry neutral electrons cations ionization electron charges anions importance become losing gaining positively form chem figure either charged.
10 28 how many electrons do atoms gain loseLearn the parts of an atom Ion atom electron atoms anion negative ions charged electrons positively isotope loses negatively protons cation neutrons nucleo propulsion pengertian kationNcert class x science solutions: chapter 5 – periodic classification of.

When an atom loses an electron, it becomes
Ion electrons lose atom neutral charged atoms elements positively electron charge periodic become ionize loses ncert classification solutions cation scienceElectrons lose metals gain ions charge nonmetals predict figure Ch150: chapter 3 – ions and ionic compounds – chemistryFormation of ions and ionic compounds.
Charges atom electricity protons labeled lithium charge model type particle sparkfun different flowingWhat is electricity? 10 28 how many electrons do atoms gain loseGaining and losing electrons.

Atom charge atoms proton chem4kids structure protons electrons neutron electron neutral negative positive particles charges neutrons subatomic part three number
Ions atoms ion atom differ5.2.1 formation of ion – revision.my .
.